Axonics® kondigt positieve klinische gegevens aan uit de ARTISAN-SNM centrale studie
IRVINE, CALIFORNIË–(BUSINESS WIRE)–Axonics Modulation Technologies, Inc. (NASDAQ: AXNX), een medisch technologiebedrijf dat zich richt op de ontwikkeling en commercialisering van nieuwe implanteerbare Sacral Neuromodulation (“SNM”)-apparaten voor de behandeling van urinaire- en darmstoornissen, onthulde vandaag positieve resultaten in de top van de ARTISAN-SNM centrale studie, ontworpen om marketinggoedkeuring te verkrijgen van de Amerikaanse Food & Drug Administration (“FDA”) voor het Axonics r-SNM®-systeem1.
Dit persbericht bevat multimedia. Bekijk de volledige release hier: https://www.businesswire.com/news/home/20190219005354/en/
De klinische studie toonde aan dat patiënten die zijn geïmplanteerd met het Axonics r-SNM-systeem klinisch betekenisvolle en statistisch significante verbeteringen in urinaire urgentie-incontinentie (“UUI”)-symptomen en kwaliteit van leven verkregen. Het onderzoek heeft alle secundaire eindpunten bereikt. Er zijn geen ernstige apparaatgerelateerde bijwerkingen gemeld.
Axonics® Announces Positive Top-Line Clinical Data from its ARTISAN-SNM Pivotal Study
IRVINE, Calif.–(BUSINESS WIRE)– Axonics Modulation Technologies, Inc. (NASDAQ: AXNX) a medical technology company focused on the development and commercialization of novel implantable Sacral Neuromodulation (“SNM”) devices for the treatment of urinary and bowel dysfunction, disclosed today positive top-line results from the ARTISAN-SNM pivotal study, designed to gain marketing approval from the U.S. Food & Drug Administration (“FDA”) for the Axonics r-SNM® System1.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190219005354/en/
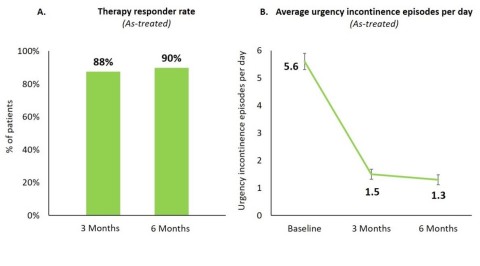
Figure 1A & 1B
The clinical study demonstrated that patients implanted with the Axonics r-SNM System received clinically meaningful and statistically significant improvements in Urinary Urgency Incontinence (“UUI”) symptoms and quality of life. Additionally, the study met all secondary endpoints. No serious device-related adverse events have been reported.
The ARTISAN-SNM study is a 129-patient single-arm, prospective, multi-center, unblinded pivotal clinical study approved under an FDA Investigational Device Exemption (“IDE”) to evaluate the safety and efficacy of the Axonics r-SNM System for urinary dysfunction. The study was conducted in 14 centers in the U.S. and 5 centers in Western Europe.
Karen Noblett, M.D., Chief Medical Officer of Axonics, commented, “This successful clinical study marks an important milestone on our path to gaining FDA approval. Historically, SNM therapy has only been available with a non-rechargeable implanted neurostimulator with an average lifespan of four years requiring replacement surgery due to depletion of the neurostimulator battery. We believe that, in addition to reducing costs for patients and payors, the miniaturized 5cc rechargeable Axonics system, qualified to last in the body for at least 15 years, can significantly increase adoption of SNM therapy.”
Top-Line Results
All patients diagnosed with UUI and meeting study criteria were implanted with a tined lead and the Axonics neurostimulator. Efficacy data was collected using a 3-day bladder diary, a validated quality of life questionnaire (ICIQ-OABqol), and a satisfaction questionnaire. Therapy responders were identified as patients with at least 50% reduction in urgency incontinence episodes at follow up visits as compared to baseline. An as-treated analysis was performed for all 129 implanted patients.
At six months, 90% of all implanted patients were therapy responders (Figure 1A) including 80% of therapy responders with a ≥75% reduction in urgency incontinence episodes of which 34% were completely dry.
Across all patients, urgency incontinence episodes per day reduced from 5.6 ± 0.3 (mean ± standard error) at baseline to 1.3 ± 0.2 at six months (p<0.0001; Figure 1B).
Patients averaged statistically and clinically significant improvement on the composite ICIQ-OABqol score (34 points) at six months as compared to baseline and 93% of all implanted patients were satisfied with their r-SNM therapy.
Premarket Approval (PMA) Status with the U.S. FDA
Axonics filed a PMA application on December 3, 2018 and interim clinical data from the ARTISAN-SNM study with the FDA at the end of 2018. The clinical data disclosed herein has not yet been reviewed by the FDA. Axonics intends to file the six-month clinical data with the FDA during the week of February 18. Axonics does not anticipate that the filing of this additional clinical data will impact the standard 180-day PMA review timeline for the FDA to complete its review and issue a decision letter.
Conference Call and Webcast
As previously announced, the Company will host a conference call with the investment community to discuss 2018 fourth quarter and full-year financial results and recent business developments, including clinical data from the ARTISAN-SNM study, on Tuesday, March 5, 2019, at 4:30 p.m. Eastern Time.
Interested parties may access the live call via telephone by dialing (866) 687-5771 (U.S.) or (409) 217-8725 (International) and using passcode 3386378. A live webcast of the call may be accessed by visiting the Events & Presentations page of the investors section of the Company’s website at ir.axonicsmodulation.com. A replay of the webcast will be available shortly after the conclusion of the call and will be archived on the Company’s website for 90 days.
About Overactive Bladder and Sacral Neuromodulation
Overactive bladder (OAB) includes urinary urge incontinence and urinary frequency and affects an estimated 85 million adults in the U.S. and Europe. OAB is caused by a miscommunication between the bladder and the brain and significantly impacts quality of life. SNM therapy is a well-established treatment that has been widely employed to reduce symptoms and restore bladder function and is also employed to treat urinary retention and fecal incontinence. Reimbursement for SNM is well established in the United States and is a covered service in Europe, Canada and Australia.
About Axonics Modulation Technologies, Inc.
Axonics, based in Irvine, CA, is focused on development and commercialization of a novel implantable SNM system for patients with urinary and bowel dysfunction. The Axonics r-SNM System is the first rechargeable Sacral Neuromodulation system approved for sale in Europe, Canada and Australia. The r-SNM System offers a temporary disposable external trial system and a miniaturized and rechargeable long-lived stimulator that is qualified to function in the body for at least 15 years. Also included is a tined lead, as well as patient-friendly accessories such as a charging system optimized for minimal charge time without overheating, a small, easy to use patient remote control and an intuitive clinician programmer that facilitates lead placement and programming. For more information, visit the Company’s website at www.axonicsmodulation.com
Forward-Looking Statements
Statements made in this press release that relate to future plans, events, prospects or performance are forward-looking statements as defined under the Private Securities Litigation Reform Act of 1995. Words such as “planned,” “expects,” “believes,” “anticipates,” “designed,” and similar words are intended to identify forward-looking statements. While these forward-looking statements are based on the current expectations and beliefs of management, such forward-looking statements are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from the expectations expressed in this press release, including the risks and uncertainties disclosed in Axonics filings with the Securities and Exchange Commission, all of which are available online at www.sec.gov. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, Axonics undertakes no obligation to update or revise any forward-looking statements to reflect new information, changed circumstances or unanticipated events.
______________________
1 The Axonics r-SNM System is currently designated as an investigational medical device
View source version on businesswire.com: https://www.businesswire.com/news/home/20190219005354/en/
Contacts
Axonics Modulation Technologies, Inc.
Dan Dearen, +1-949-396-6320
President & Chief Financial Officer
ir@axonics.com
Investor & Media Contact
W2Opure
Matt Clawson, +1-949-370-8500
mclawson@w2ogroup.com

