Ultivue breidt UltiMapper ™ -portfolio van multiplexmarkertests uit voor uitgebreide fenotypering van de immuunmicro-omgeving en weefsel Immuno-Profiling-onderzoek
CAMBRIDGE, Mass. – (BUSINESS WIRE) – Ultivue is een toonaangevende ontwikkelaar van identificatie en kwantificeringstests voor de weefselbiomarkers voor translationeel onderzoek en pathologielabs. Vandaag heeft Ultivue de uitbreiding aangekondigd van zijn UltiMapper ™ multiplex immunofluorescentiekitportfolio met een T-reg en MDSC-kit. De UltiMapper I / O T-reg-set identificeert regulatoire T-cellen en cytotoxische T-cellen binnen de tumorcontext en maakt fenotypering van geactiveerde dubbel-positieve T-cellen mogelijk. De UltiMapper I / O MDSC-kit maakt de identificatie en karakterisering van myeloïde afgeleide suppressorcellen mogelijk en stelt onderzoekers in staat om M-MDSC te onderscheiden van PMN-MDSC-fenotypes.
Dit persbericht bevat multimedia. Bekijk hier de volledige release: https://www.businesswire.com/news/home/20191106005226/en/
Ultivue Expands UltiMapper™ Portfolio of Multiplex Marker Assays for Comprehensive Phenotyping of the Immune Microenvironment and Tissue Immuno-Profiling Research
CAMBRIDGE, Mass.–(BUSINESS WIRE)– Ultivue, a leading developer of tissue biomarker identification and quantification assays for translational research and pathology labs, today announced the expansion of its UltiMapper™ multiplex immunofluorescence kit portfolio with a T-reg and MDSC kit. The UltiMapper I/O T-reg kit identifies regulatory T cells and cytotoxic T cells within the tumor context and allows for the phenotyping of activated double positive T cells. The UltiMapper I/O MDSC kit enables the identification and characterization of myeloid derived suppressor cells and allows researchers to differentiate M-MDSC from PMN-MDSC phenotypes.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191106005226/en/
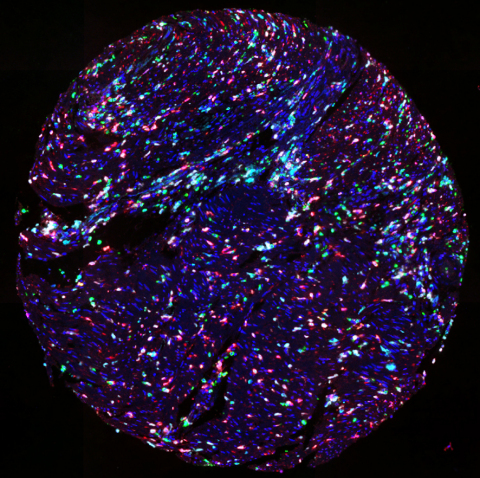
TMA of colorectal cancer stained with UltiMapper I/O MDSC kit (CD11b green, CD14 red, CD15 magenta, HLA-DR cyan) (Photo: Business Wire)
The new kits have the same benefits of high-throughput whole-slide imaging using conventional IHC equipment and workflows as the previously released UltiMapper I/O PD-L1, UltiMapper I/O PD-1, UltiMapper I/O T-act, and UltiMapper I/O APC kits. “The addition of the T-reg and MDSC kits to the UltiMapper platform now provides researchers with an in-depth view of the sources of overall cell regulation and suppression critical in characterizing the signatures at play within the tumor microenvironment,” said Philippe Mourere, Senior VP of Commercial Operations at Ultivue. “We are very encouraged that the increased breadth and depth of immuno-profiling markers in tissue samples will support further validation of clinically-relevant biomarkers in immuno-oncology, inflammation & immunology, and cancer research.”
Research featuring data generated by the MDSC and T-reg kits will be presented in three of a total of ten posters showcasing the Ultivue technology at the upcoming Society for Immunotherapy of Cancer (SITC) Annual Meeting at The Gaylord National Resort & Convention Center in National Harbor, MD, November 6-10.
Details of the poster sessions can be found below:
Friday, November 8, 7am – 8pm:
P39: Looking beyond the assay: Comparison of multiplex chromogenic and fluorescent IHC for standardized immune oncology profiling in non-small cell lung carcinoma patients
P45: Multiplex IF staining, whole-slide imaging, and spatial phenotyping of T-cell exhaustion, T-regs, and MDSCs in Tumor FFPE samples
P49: Use of Ultivue ISP mIF to localize and quantify T-regs in human FFPE tissue
P55: Combining the best of 2 worlds: Transfer of mIF images from NSCLC patients into pseudo-chromogenic mIHC images
P127: Development of a 12-plex mIF panel for in depth investigation of the TIME landscape analyzing 4,096 phenotypes
P563: Profiling T-reg and CTL relationships in the TME of NSCLC FFPE samples
P567: Characterizing MDSCs in the TME of CRC FFPE samples
Saturday, November 9, 7am – 8:30pm:
P40: Tumor immunity signatures to expand current Dx approaches in MMR-deficient cancers in the context of Lynch Syndrome through ISP technology and Tissue Phenomics integration
P46: Same-slide mIF and brightfield histological staining as a new research tool for fast and comprehensive pathology assessment of the TME
P70: Tissue-based characterization of T-cell exhaustion in IBD and CRC using mIHC
Ultivue’s scientific team welcomes the opportunity to interact with the biomedical community during the SITC Annual Meeting at booth #428.
About Ultivue
By developing a single set of novel, proprietary reagents used both for biomarker discovery (higher content, low throughput) and translational use (lower content, high throughput), Ultivue is connecting the insights gained from research directly into the pathology lab. Ultivue’s UltiMapper multiplexed assays applied to tissue biopsy samples enable simultaneous quantitation of multiple biomarkers with sub-cellular spatial resolution and fit completely within traditional IHC workflows. Translational and clinical researchers leverage UltiMapper assays to elucidate complex biology and demonstrate their clinical utility as precision medicine research tools. Ultivue is expanding its UltiMapper assay product portfolio and menu of contract research services to provide a comprehensive set of precision medicine solutions for oncology research and focus in other therapeutic areas.
Ultivue is based in Cambridge, MA. For more information, visit www.ultivue.com
For Research Use Only. Not for use in diagnostic procedures.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191106005226/en/
Contacts
Louis Levy
Director, Business & Corporate Development
+1-617-945-2662
